TECHNOLOGY
What is the density of silver in grams
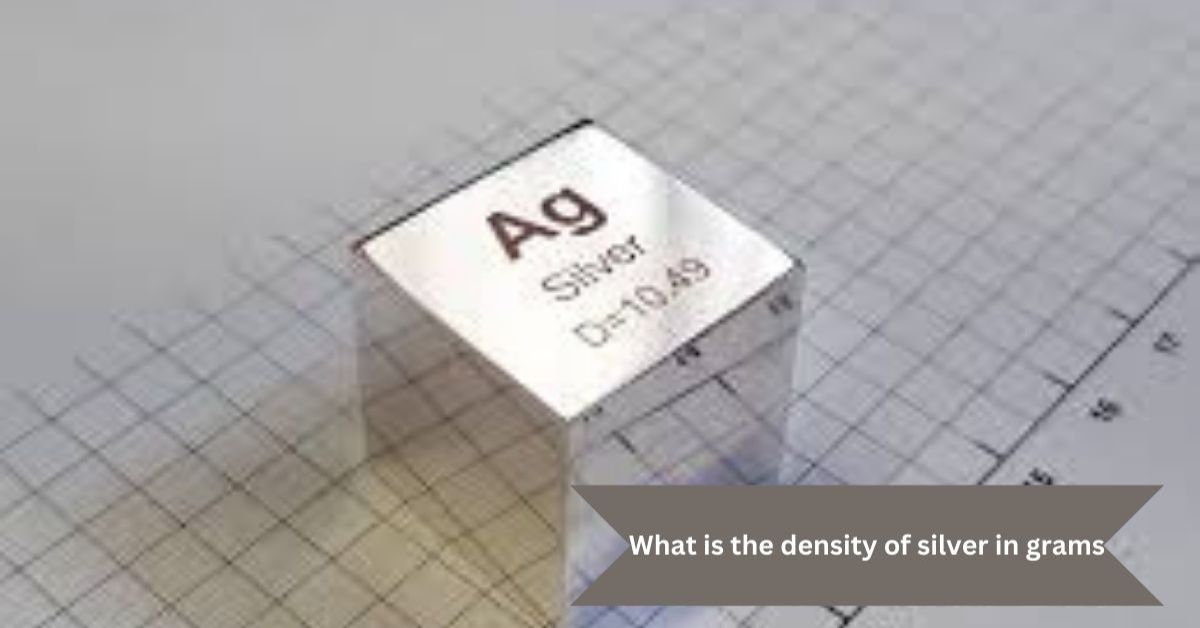
What is the Density of Silver in Grams? Understanding Its Properties
Silver is a precious metal widely recognized for its beauty, conductivity, and usefulness in various applications, from jewelry to industrial uses. One of the essential physical properties of silver that plays a significant role in its applications is its density. The density of silver is a key factor in determining its weight, volume, and how it behaves under different conditions. In this article, we will explore what density is, how the density of silver is measured, and its relevance in various industries.
What is Density?
Density is a physical property that describes how much mass is contained in a given volume of a substance. It is calculated using the formula:
Density=MassVolumeDensity = \frac{Mass}{Volume}
Where:
- Mass is the amount of matter in an object, typically measured in grams (g) or kilograms (kg).
- Volume is the amount of space that an object occupies, usually measured in cubic centimeters (cm³) or liters (L).
The density of a material indicates how compact or spread out its particles are. For example, materials with high density have tightly packed particles, making them heavier for their size. Conversely, low-density materials have more spaced-out particles, making them lighter.
The Density of Silver: A Key Physical Property
The density of silver in grams is an important factor in understanding its characteristics and uses. Silver has a relatively high density compared to many other metals, which makes it ideal for specific applications. The density of silver is approximately 10.49 grams per cubic centimeter (g/cm³) at room temperature (20°C or 68°F). This means that for every cubic centimeter of silver, it weighs about 10.49 grams.
How Is the Density of Silver Measured?
The density of a substance like silver is determined through various methods, but the most common is by measuring its mass and volume. Here’s how the process typically works:
-
Measuring Mass: The mass of a sample of silver is measured using a precise balance. The mass is usually given in grams (g).
-
Measuring Volume: The volume of silver can be determined by using geometric measurements for regular shapes, like a silver cube, or by employing water displacement for irregularly shaped objects. In the case of water displacement, a known amount of water is placed in a container, and the silver object is submerged. The volume of displaced water corresponds to the volume of the silver object.
-
Calculating Density: Once both the mass and volume are determined, density can be calculated by dividing the mass by the volume.
Factors Affecting the Density of Silver
While the density of pure silver is generally consistent, certain factors can slightly alter its density:
1. Temperature:
- Silver, like all metals, expands when heated and contracts when cooled. This means that the density of silver can change with temperature. At higher temperatures, the volume of silver increases, causing a slight decrease in its density.
- The effect of temperature on density is relatively small, but it is still important in precision applications, especially in industries where silver is used in high-heat environments.
2. Impurities:
- Pure silver, or 999 fine silver, has a consistent density. However, silver alloys, such as sterling silver (92.5% silver and 7.5% other metals like copper), will have a slightly different density due to the presence of other materials.
- Alloys tend to have a lower density than pure silver because the added metals may be less dense than silver itself.
3. Manufacturing Process:
- The way silver is processed and shaped can also affect its final density. For instance, silver that is hammered or rolled out into thin sheets may experience slight changes in density due to internal stresses and crystal structure changes.
Why Is the Density of Silver Important?
Understanding the density of silver is important in various industries and applications. Below are some of the key reasons why this property is significant:
1. Jewelry and Coinage
- Weight and Value: Silver’s density plays a crucial role in determining the weight of jewelry and coins. The higher the density, the heavier the piece will be for the same volume. This is important in determining both the cost and the perceived value of silver-based items.
- Design and Durability: The density of silver affects its malleability and ability to hold intricate designs. Silver’s relatively high density allows it to be shaped into fine jewelry without being too fragile.
2. Industrial Applications
- Conductivity: Silver is known for its excellent electrical and thermal conductivity, which is influenced by its atomic structure. The density of silver contributes to its conductivity by affecting how freely electrons move through the material.
- Electronics and Soldering: Due to its high density and conductivity, silver is widely used in high-performance electronics and in the creation of solder materials for electronic components.
3. Weighting and Calibration Instruments
- The density of silver in grams is critical in calibration instruments and other equipment that require highly accurate measurements of mass and weight. Knowing the density of silver allows manufacturers to use it as a reference material for precision scales and balances.
4. Art and Silverware
- Silver’s density contributes to the perception of quality and luxury in high-end art pieces, silverware, and collectibles. Heavier silver items often feel more substantial and valuable, making the density an important consideration for artists and craftsmen.
Comparing Silver Density to Other Metals
Silver’s density of 10.49 g/cm³ is relatively high compared to many other common metals. Here’s how it compares to some other well-known metals:
| Metal | Density (g/cm³) |
|---|---|
| Silver | 10.49 |
| Gold | 19.32 |
| Copper | 8.96 |
| Aluminum | 2.70 |
| Platinum | 21.45 |
| Iron | 7.87 |
As you can see, gold and platinum have higher densities than silver, which makes them heavier for the same volume. On the other hand, copper, aluminum, and iron have lower densities than silver, which is why they are lighter and less valuable on a per-gram basis.
Applications of Silver Based on Its Density
The density of silver has important implications in various applications, including:
-
Silver Coins and Bullion: Due to its density and value, silver is widely used in the production of coins and bullion. The mass and volume of silver coins are essential in determining their worth.
-
Thermal and Electrical Conductivity: In industries where high conductivity is required, silver’s density is a contributing factor to its effectiveness in applications like electrical wiring, conductors, and heat exchangers.
-
Medical Devices: Silver’s density and biocompatibility make it useful in medical devices and equipment, such as wound dressings and surgical instruments.
-
Photography: Silver compounds were historically used in photographic film, owing to their density and light-sensitive properties. Although digital photography has replaced much of this, silver’s role in the industry is still significant.
Conclusion
The density of silver, which is approximately 10.49 g/cm³, plays a significant role in its many applications, from jewelry to industrial uses. This essential physical property is influenced by factors such as temperature, impurities, and manufacturing processes, and understanding it is crucial for industries that rely on silver’s unique properties. Whether it’s for its weight in coinage, its conductivity in electronics, or its durability in fine jewelry, the density of silver is a defining characteristic that shapes its value and utility.
TECHNOLOGY
How Insetprag Transforms Prague Pest Control

Pests have always been a problem, but in recent years, rising global temperatures, increased travel, and urban density have made insect infestations more common and harder to control. Traditional chemical sprays often bring more harm than good, leading people to look for environmentally safe repellents that won’t damage ecosystems.Insetprag represents this shift. It’s not just about exterminating pests; it’s about balancing prevention, removal, and sustainable solutions.
Imagine living in Prague during summer: mosquitoes thrive near the Vltava River, ants sneak into cafes, and bed bugs occasionally spread through hotels. For locals and tourists alike, reliable Prague pest control services are a necessity. Insetprag aims to provide comprehensive, eco-conscious, and technologically advanced options.
The Evolution of Insect Control Solutions
From Chemicals to Eco Pest Management
In the past, chemical pesticides dominated the pest control industry. While they were effective, they left behind toxic residues that harmed soil, water, and non-target species. Today, eco pest management is leading the way.
Biological controls: Using natural predators like ladybugs to combat aphids.
Essential oil sprays: Safer, plant-based alternatives for household use.
Integrated Pest Management (IPM): Combining prevention, monitoring, and targeted treatment.
The benefit? Long-term effectiveness without the collateral damage.
Insect Repellent Devices That Work Smarter
One of the biggest shifts in 2025 has been the rise of insect repellent devices powered by AI and IoT. These gadgets not only repel insects but also collect data on pest activity.
Some of the most popular innovations include:
Ultrasonic repellents that use sound waves to deter pests.
Smart bug zappers with energy-efficient LED lights.
Wearable repellents, perfect for outdoor activities.
These devices reduce the need for harmful sprays while providing continuous household insect protection.
Prague Pest Control Services: Local Expertise Meets Innovation
If you live in Prague, you’ve likely noticed an increase in professional pest control services offering modernized packages. Traditional exterminators now combine insect removal services with smart monitoring systems.
For instance:
A family in Prague 7 installed a smart pest control system that automatically detects bed bugs in furniture.
Restaurants in Old Town are using bug trap technology that integrates with mobile apps to alert managers about cockroach activity before it becomes a full infestation.
This integration of traditional expertise with modern technology makes Prague a leader in urban pest management.
The Science Behind Insect Prevention Methods
Prevention has always been better than cure. But in 2025, insect prevention methods have become more advanced, combining design, materials, and data-driven insights.
Smart window screens that release micro-doses of repellents.
Soil-based solutions that discourage ants from nesting near homes.
Moisture control systems that reduce environments where mosquitoes breed.
By adopting these methods, homeowners can drastically reduce their dependence on chemical sprays.
Bug Trap Technology: A Closer Look
Bug trap technology has gone beyond sticky boards and flypaper. In 2025, traps are smarter, more targeted, and environmentally conscious.
Pheromone traps attract specific insects like moths or beetles.
AI-powered traps analyze captured insects to provide prevention tips.
Solar-powered traps offer a sustainable solution for outdoor use.
One user recently tweeted: “Never thought I’d geek out over a bug trap, but my smart insect catcher just pinged me with data about mosquito activity in my backyard. This is the future!”
Smart Pest Control Systems: Automation at Its Best
Smart homes are no longer just about lighting and thermostats—they now include smart pest control systems. These systems integrate with home assistants like Alexa or Google Home to provide updates on insect activity.
Automatic scheduling of repellents.
Real-time alerts about unusual pest behavior.
AI-driven recommendations for prevention.
This level of automation brings peace of mind, especially for households with children and pets.
Household Insect Protection in Everyday Life
Let’s talk practicality. What does household insect protection actually look like for a typical family?
Kitchen hygiene: Smart bins that seal automatically to prevent flies.
Bedroom defense: Anti-mosquito smart screens and essential oil diffusers.
Garden care: Eco-friendly sprays that don’t harm pollinators.
With these steps, homes remain comfortable without sacrificing safety or sustainability.
The Rise of Insect Removal Services
While prevention is ideal, infestations still happen. That’s where professional insect removal services step in. In 2025, these services are more customer-friendly, data-driven, and eco-conscious.
On-demand apps allow you to book pest control within hours.
Services guarantee environmentally safe repellents instead of harsh chemicals.
Real-time tracking shows when and where technicians treated your property.
This makes pest control less intimidating and far more transparent.
Balancing Innovation with Environmentally Safe Repellents
Perhaps the biggest challenge in pest management has always been safety. Harsh pesticides can kill pests, but they often harm beneficial insects like bees. That’s why environmentally safe repellents are at the heart of modern solutions.
Examples include:
Neem oil sprays.
Citronella candles.
Non-toxic gels for cockroaches.
These options strike the balance between effectiveness and ecological responsibility.
Real-Life Example: Insetprag in Action
In insetprag, a café owner faced recurring issues with fruit flies near their bar. Instead of relying on chemical sprays, they installed a smart bug trap technology system combined with eco pest management techniques like regular waste monitoring and herbal repellents. Within two weeks, the fly population dropped by 80%, without any disruption to customers.
The owner said: “It was a relief to find something that worked without risking my staff’s health or the taste of our cocktails.”
Pros and Cons of Modern Pest Control
Pros
Eco-friendly and safe for children/pets.
Data-driven and preventive.
More affordable in the long run.
Cons
Higher upfront costs for smart devices.
Some eco repellents need frequent reapplication.
Dependence on apps may feel overwhelming for older users.
FAQ’s
How effective are eco pest management solutions compared to chemical sprays?
Eco pest management is just as effective in the long term because it focuses on prevention, monitoring, and targeted solutions. While chemical sprays may give quick results, eco methods prevent re-infestation and avoid harmful side effects.
Can smart pest control systems replace traditional insect removal services?
Not entirely. Smart systems are excellent for prevention and monitoring, but when infestations get severe, professional insect removal services are still necessary.
Are insect repellent devices safe for kids and pets?
Yes, most modern insect repellent devices are designed with safety in mind, using ultrasonic waves or natural repellents instead of toxic chemicals.
What’s the best insetprag prevention method for city apartments?
For urban living, Prague pest control services recommend sealing entry points, installing smart screens, and using eco-friendly indoor repellents.
Conclusion
Insetprag isn’t just a product—it’s a movement toward smarter, safer, and more sustainable pest management. With innovations in bug trap technology, smart pest control systems, and environmentally safe repellents, we’re entering a new era of household and commercial protection.
TECHNOLOGY
Top 10 Besten Machines for Industrial Use

Understanding the Besten company profile helps explain why the brand is growing in visibility. Founded with a mission to provide affordable yet high-quality equipment, Besten machines focuses on both industrial-scale projects and consumer-level needs.
In recent years, the company has expanded its portfolio from construction tools to cleaning equipment and industrial machines. Customers often highlight the balance between durability and cost-effectiveness, making it a popular choice in competitive markets.
A contractor once put it this way: “Besten machines don’t always have the fanciest designs, but they work hard, last long, and don’t break the bank.”
Besten Machine Reviews: What Customers Are Saying
Common Strengths Reported
- Durability: Many users find that Besten equipment holds up well in heavy use.
- Affordability: Positioned between budget and premium brands, giving good value.
- Ease of Use: Most models are designed with straightforward functionality.
Reported Limitations
- Limited spare parts in certain regions.
- Customer support response times vary.
- Design may feel basic compared to premium competitors.
Overall, Besten machine reviews suggest a solid mid-tier brand with practical reliability.
Besten Equipment Categories
Besten Industrial Machines
The Besten has steadily gained traction with industrial machines for factories and large-scale operations. These include cutting, welding, and heavy-duty handling tools. Users report they’re best suited for medium-sized businesses that need quality without premium prices.
Besten Construction Tools
For contractors and builders, Besten construction tools have become an accessible choice. From power drills to portable mixers, the emphasis is on sturdy build and consistent output. They may not rival high-end global brands, but for regional projects, they’re considered reliable workhorses.
Besten Cleaning Machines
This is where the brand has made waves recently. Besten cleaning machines—vacuum systems, floor polishers, and pressure washers—are widely adopted by small cleaning businesses. The balance of price and efficiency makes them especially appealing for startups.
Popular Besten Products in 2025
Here’s a quick look at Besten products users mention most often:
| Product Type | Popular Model | Ideal For | Price Range (USD) |
|---|---|---|---|
| Industrial Machine | B-IND3000 | Medium factories | $1,200–$2,000 |
| Construction Tool | B-CON1500 | Contractors/Builders | $400–$800 |
| Cleaning Machine | B-CL1000 | Small cleaning businesses | $250–$500 |
| Consumer Tool | B-HOME500 | DIY/Home use | $120–$250 |
Besten Machine Dealers: Where to Buy Safely
One challenge buyers face is finding trusted Besten machine dealers. Since counterfeit equipment occasionally surfaces in certain markets, it’s important to:
Buy from authorized dealers listed on the official company site.
Ask for warranty certificates at purchase.
Check whether the dealer stocks Besten spare parts for future needs.
Besten Machine Warranty and After-Sales Support
A Besten machine warranty typically covers:
- 12–24 months on parts and labor.
- Extended warranty options for industrial equipment.
- Limited coverage for wear-and-tear items.
Customers report that while warranty policies are fair, support responsiveness can vary by region. That’s why purchasing through recognized Besten dealers makes a difference.
Availability of Besten Spare Parts
Maintenance is key to extending machine life. Besten spare parts availability is decent in urban markets but can be harder to source in remote regions. Many buyers recommend ordering original parts directly through authorized dealers to avoid counterfeit risks.
Why Choose Besten Machines Over Competitors?
- Affordability: Positioned as mid-range, offering quality without premium pricing.
- Product Range: From industrial machines to cleaning tools, covering multiple industries.
- Ease of Use: Less complexity compared to premium brands, great for new businesses.
- Warranty Coverage: Reasonable support for the price point.
But: If you need ultra-advanced features or cutting-edge designs, you might prefer higher-end brands.
Real-Life Example: A Small Business Perspective
A cleaning service startup in Southeast Asia shared: “We started with just two Besten cleaning machines. They were affordable, easy to maintain, and reliable for daily jobs. As our business grew, we scaled with more advanced equipment, but Besten gave us the foundation to get started.”
This story highlights how Besten machines help new businesses grow without massive upfront investment.
Comparing Besten to Other Brands
| Feature | Besten Machines | Premium Global Brands | Low-Cost Brands |
|---|---|---|---|
| Price Range | Mid-range | High | Budget |
| Durability | Good | Excellent | Variable |
| Features/Tech | Standard | Advanced | Basic |
| Spare Parts Availability | Moderate | Wide | Limited |
| Best For | Small/Medium business | Enterprises | DIY/Short-term |
Risks and Considerations
- Counterfeit Products: Only buy from official dealers.
- Warranty Coverage: Varies regionally, always confirm before purchase.
- Spare Parts: Can be an issue in less developed markets.
The Future of Besten Equipment in 2025 and Beyond
With global demand for cost-effective industrial and cleaning equipment, Besten is expected to expand into smart machinery integration. AI-driven diagnostics and IoT-enabled monitoring may soon feature in new product lines, making the machines smarter and easier to maintain.
FAQ’s
Are Besten machines reliable for industrial use?
Yes, Besten industrial machines are considered reliable for medium-scale industries, though not as feature-rich as premium global brands.
Where can I buy authentic Besten equipment?
Always purchase from authorized Besten dealers to ensure warranty support and genuine spare parts availability.
Do Besten cleaning machines work for commercial businesses?
Yes, they’re popular with small cleaning companies thanks to their affordability and ease of use.
How does the Besten warranty compare to others?
It’s competitive in the mid-range segment, covering most parts for 1–2 years, though coverage can vary by region.
Conclusion
If you’re looking for reliable, mid-range equipment that balances cost and durability, Besten machines are worth considering. They’re especially ideal for small to medium businesses in construction, cleaning, or light industrial sectors.
TECHNOLOGY
From Downtime to Uptime: How IT Managed Services Boost Business Efficiency

Introduction to IT Managed Services
Minimizing downtime is crucial for maintaining business continuity and efficiency in the digital landscape. Many businesses today turn to IT managed service providers and beyond to keep their systems running smoothly. These services allow companies to delegate the maintenance of IT infrastructure to specialists, freeing up resources to focus on core operations. This shift not only alleviates the burden of dealing with unexpected technical issues but also ensures that businesses can leverage the latest technological advancements without the heavy lifting often required by in-house teams.
The Impact of Downtime on Business Efficiency
Downtime has a tangible impact on productivity and can severely damage a company’s reputation. A Gartner study indicated that the average cost of IT downtime is $5,600 per minute. This underscores the need for robust IT solutions to minimize disruptions and maximize business output. When systems are down, employee resources are underutilized, and customer satisfaction can plummet. In sectors like finance or healthcare, where data accessibility and reliability are paramount, even a momentary lapse can lead to substantial losses, both financially and in terms of customer trust. In urban centers with dense business activity, such as Toronto, many organizations turn to IT managed service providers Toronto relies on to implement preventive strategies and maintain operational continuity. These providers are often sought for their proximity and understanding of local infrastructure challenges. However, the decision to partner with a provider should still be based on thorough evaluation, including service scope, response times, and alignment with organizational needs.
How IT Managed Services Minimize Downtime
Managed IT services proactively monitor and manage networks to prevent downtime. By regularly updating systems and troubleshooting potential issues, these services minimize disruptions. Continuous monitoring allows for early detection of anomalies, ensuring swift corrective actions that keep businesses operational. With real-time monitoring, managed services can address minor issues before they escalate into major problems. This proactive stance preserves business resources and enhances operational resilience, providing a more stable environment for employees and clients.
Proactive vs. Reactive IT Support
Proactive IT support focuses on anticipating and preventing issues before they occur rather than addressing them after they arise. This approach significantly reduces the frequency and severity of downtime incidents, saving businesses time and money in the long run. Unlike reactive support, which often results in scrambling to fix issues after they’ve caused a significant impact, proactive support implements solutions to potential problems before they disrupt business operations. It involves a strategic approach, including regular system audits, predictive maintenance, and consistent performance monitoring.
Cost Benefits of Outsourcing IT Services
Outsourcing IT needs can be financially advantageous. Unlike maintaining a full-time, in-house IT department, engaging managed services curtails overhead expenses. A report from Computerworld highlights how outsourcing enables access to a broad pool of IT expertise at a fraction of the cost. By outsourcing, companies can convert fixed IT expenses into variable costs, improving flexibility and budgeting. Additionally, outsourcing offers scalability options that can benefit businesses experiencing rapid growth or restructuring.
The Future of IT Managed Services in Business
Technological advancements are poised to accelerate the evolution of IT managed services. Artificial intelligence and machine learning are paving the way for more precise predictive analytics, which enhance the ability to foresee and address technical issues before they affect business processes. Integrating these technologies into IT services allows for automatic system updates, security enhancements, and a more strategic allocation of IT resources. As these tools evolve, businesses can expect even more sophisticated service offerings that support innovation, drive efficiency, and mitigate risks.
How to Choose the Right IT Managed Service Provider
Selecting the right managed service provider involves examining their industry experience and resource availability. Reviewing case studies and client testimonials can provide insights into their effectiveness. Businesses should seek providers that align with their strategic goals to ensure a fruitful partnership. It’s important to consider the provider’s approach to customer service, their ability to tailor solutions to your needs, and their track record in handling similar business environments. This careful vetting process ensures that companies find a partner capable of delivering lasting value and sustainable business growth.
-

 GENERAL8 months ago
GENERAL8 months agoClassroom6x: Revolutionizing the Future of Learning
-

 ENTERTAINMENT8 months ago
ENTERTAINMENT8 months agoUnveiling the Mystery of Kashito_Toto: A Digital Frontier
-

 TECHNOLOGY8 months ago
TECHNOLOGY8 months agoUnlocking the Power of SSIS 816: A New Era in Data Integration
-

 TECHNOLOGY8 months ago
TECHNOLOGY8 months agoUnlocking the Mystery of Vy6ys: A Hidden Gem
-

 GENERAL8 months ago
GENERAL8 months agoUnraveling Time: What Hour Was It 8 Hours Ago?
-

 GENERAL8 months ago
GENERAL8 months agoQuid Pro Quo Harassment: What It Is and Why It Matters
-

 BUSNIESS8 months ago
BUSNIESS8 months agoWhat Does ‘In Transit’ Mean? Understanding Shipment Status
-
 BUSNIESS8 months ago
BUSNIESS8 months agoUnderstanding Sell in Trade Locker: A Key to Smart Trading
